Nitrogen is one of the most important elements found on earth, and is one of the most essential nutrients taken by living organisms. Nitrogen is the major component found in the air that we breath, however in this form it is not accessible to the majority of living organisms. Approximately 78% of the atmosphere is comprised of this colorless, odorless, nontoxic gas. Nitrogen gas cannot be directly used by plants and therefore needs to be converted into nitrate compound by nitrogen fixing bacteria in soil.
The process through which this element becomes accessible for living organisms to absorb it is known as the nitrogen cycle.
Nitrogen is found in two major forms in the atmosphere. The first of these is N2, a molecule comprised of two nitrogen atoms. This format is inaccessible to the majority of organisms on earth, which makes it quite a valuable and rare resource which can hold many ecosystems back from growth. The other form is NH3, a molecule comprising a nitrogen atom and three hydrogen atoms, which is more commonly known as ammonia.
There are many other variations of nitrogen found in the world, be they man-made or organic. Because of this, nitrogen is a versatile and plastic element which sees many changes throughout the nitrogen cycle. The majority of the changes experienced by nitrogen in this cycle (for example: nitrification, ammonification, denitrification and fixation) are dependent mostly on small organisms such as bacteria and fungi.
The Processes of the Nitrogen Cycle
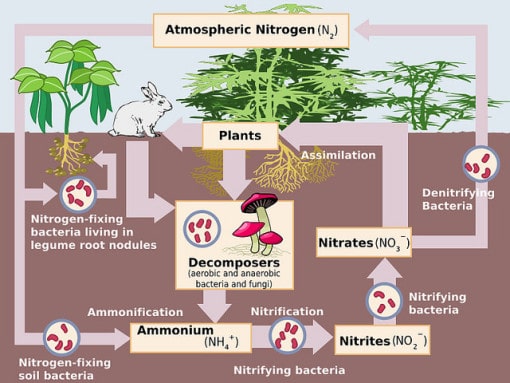
The nitrogen cycle is split up into five main processes. These processes are nitrogen fixation, assimilation, ammonification, nitrification, and denitrification. Each of these play an important role in movement of nitrogen through the different ecosystems on earth.
Nitrogen Fixation
- Nitrogen gas is incredibly abundant in the atmosphere, comprising nearly 78% of the atmosphere. However, this gas is unusable for the majority of the world’s organisms. In order for organisms to absorb nitrogen, it first needs to be converted into another chemical form. This is achieved through an organic process known as nitrogen fixation.
- A special group of micro-organisms known as prokaryotes are responsible for the majority of the fixation that occurs in nature. The process of breaking the chemical bonds found in nitrogen is one that requires a great deal of energy, and prokaryotes have evolved to be specialized to undertake this operation. Of course, humans have found other ways to achieve this result using industrial processes.
- Many plants and organisms actually have a symbiotic relationship with organisms that are specialized to fixate nitrogen. Some examples include legumes such as clover and peas, which host bacteria which are specialized to fix nitrogen. Other organisms, such as prokaryotes, are free living and do not depend on any other organisms.
- Once these plants and organisms have absorbed the nitrogen into their system with the help of these specialized micro-organisms, they are then consumed by other organisms such as herbivorous creatures and humans.
Assimilation
- Nitrogen-fixing bacteria produces ammonium that is taken up by the host plant or another soil organism and incorporated into proteins. Plants take this nitrogen from soil through their roots. Nitrogen that is obtained from animals can be traced back to the eating of plants at some stage of food chain.
- When organisms at the top of the food chain eat, they take nitrogen that has been fixed by nitrogen-fixing bacteria.
Ammonification
- Ammonification is the process by which the nitrogen found in the tissues of an organism are released when said organism dies or excretes waste. The waste or dead tissue are decomposed by micro-organisms and fungi who then release ammonia into the ecosystem. This process of converting organic nitrogen to ammonification or mineralization is an essential part of making ammonia usable for plants and other micro-organisms.
Nitrification
- Nitrification is an essential part of the nitrogen cycle which sees ammonia being converted to nitrite and then to nitrate. This is almost exclusively carried out by organisms like prokaryotes. The process comprises two important steps which have to be carried out by these specialized organisms.
- The first step is to convert ammonia to nitrite, which is a process of oxidation. This is performed by small microbes which are known simply as ammonia-oxidizers. By making use of an intermediate known as hydroxylamine – the use of enzymes called ammonia monooxygenase and hydroxylamine oxidoreductase to oxidize the ammonia in their system. The nitrogen can also be used as energy for these organisms to produce carbon from carbon dioxide.
- The second step includes oxidizing the nitrite created in the first step into nitrate. The prokaryotes that carry out this step are known as nitrite-oxidizing bacteria, and consist of examples such as Nitrospira and Nitrococcus. This step is similar to the first step, and takes an incredible amount of energy, which means that these organisms are very slow-growing. Once both steps are complete, the nitrification of the ammonia is complete.
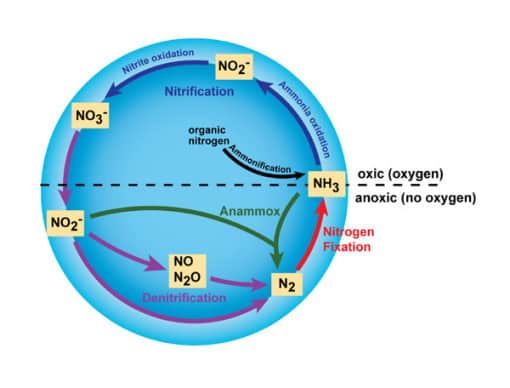
Denitrification
- Denitrification is another very important process in the nitrogen cycle, and it comprises the conversion of nitrate into nitrogen gas. This is the process by which the nitrogen that is found in forms usable by organisms is returned to the atmosphere to be redistributed. The major product of denitrification is dinitrogen gas (N2), however it can also result in the production of gases such as nitrous oxide (N2O), which is widely considered to be a pollutant gas that is contributing to global warming.
- Nitrification is a process which mostly happens in soils and bodies of water, meaning that it is a process that typically does not require oxygen. Like most other parts of the nitrogen cycle, denitrification is carried out by prokaryotes. These organisms use organic carbon to generate the energy to denitrify the organic nitrogen.
- The process of denitrification removes the nitrate and nitrite from ecosystems and returns it to the atmosphere as dinitrogen gas, which is the form in which it is most abundant. From here, the redistributed nitrogen is subjected to the other steps in the cycle in order to keep it moving from ecosystem to ecosystem, fuelling the growth of organisms around the world.
How Increase in Nitrogen Affects Ecosystem?
- Like most other natural cycles and process, the nitrogen cycle is severely disrupted by human activity. The use of nitrogen-based fertilizers and the burning of fossil fuels increase the amount of biologically usable nitrogen found the ecosystem at any one time. Nitrogen is often an important limiting factor for ecosystems, and so human activity increasing the fixation of nitrogen into usable nitrogen has a profound effect upon the growth of ecosystems the world over.
- Too much nitrogen can actually poison many ecosystems, leading to imbalances in the nutrients found in plants and trees, which has a profound effect not only upon the nitrogen cycle and the ecosystem, but also upon the carbon cycle.
- Similarly, over-abundance of fixed nitrogen found in soil can cause the nitrate to leak into water-based ecosystems, as well as finding its way into the drinking water of humans and animals alike. The increase in nitrogen can lead to reductions of the oxygen levels in water, causing the death of flora and fauna alike. It can stimulate the growth of harmful algae and can dramatically alter the balance of the ecosystem as a whole.