If you pay attention to the climate talks, you have probably heard concern expressed over the rising rates of ocean acidification. In a nutshell, this means that the pH levels within the ocean are becoming more acidic. This has happened before in the Earth’s history, but it didn’t affect mankind as they weren’t in existence. Now, ocean acidification holds the potential for impacting the human race in a great way.
According to Wikipedia,
“Ocean acidification is the ongoing decrease in the pH of the Earth’s oceans, caused by the uptake of carbon dioxide (CO
2) from the atmosphere. Seawater is slightly basic (meaning pH > 7), and ocean acidification involves a shift towards pH-neutral conditions rather than a transition to acidic conditions (pH < 7).”
Ocean acidification – the excess carbon dioxide in the atmosphere that is turning the oceans increasingly acid – is a slow but accelerating impact with consequences that will greatly overshadow all the oil spills put together. The warming trend that is CO2-related will overshadow all the oil spills that have ever occurred put together.
~ Sylvia Earle
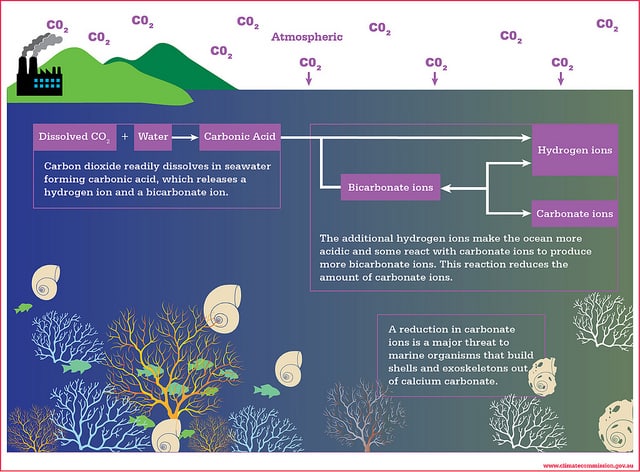
Ocean acidification or “OA” for short is caused by an increase in carbon dioxide in the water. This then increases the acid pH of the water and prohibits calcification. The reason this is so detrimental is that too much carbon dioxide has the effect of blocking photosynthesis.
Many people don’t think about photosynthesis when it comes to the ocean, but light penetrates the ocean and affects all forms of marine and plant life within the ecosystem. If the light is blocked, certain species begin to die out. Ocean acidification affects bottom-dwelling organisms, algae and coral mostly. That, in turn, has a chain reaction effect that, in the end, affects ground-dwelling life.
Earth’s oceans have maintained stable acidity levels over the last tens of millions of years. It’s within this environment that flora and fauna have risen and flourished. Over the last 100 years, there has been an unprecedented increase in the number of fossil fuel-powered vehicles, industries and power plants.
The unfortunate consequence, however, has been the emission of billions of tons of CO2 into the atmosphere. Due to the emission of billions of tons of carbon dioxide (CO2) and other greenhouse gases into the Earth’s atmosphere, the ocean has absorbed some 525 billion tons of CO2.
The oceans currently absorb at least a quarter of the CO2 that gets generated by burning fossil fuels. It is estimated that oceans absorb CO2 approximately 22 million tons per day. As 70% of the earth’s surface is covered with water, enough CO2 can have a major impact. The absorption of a high amount of CO2 per day has resulted in the dropping of pH in surface waters.
Initially, it appeared to scientists that it is helping the environment as it leaves less CO2 in the atmosphere as more of it could have caused catastrophic effects on the climate. But, in the last decade, scientists realized that when CO2 gets dissolved in the ocean, the water becomes more acidic, and the ocean’s pH drops.
pH is a measure of how acidic or basic the ocean is. The introduction of a large amount of CO2 into the ocean is affecting the life cycle of many marine plants and animals and altering the water cycle.
Over the last 300 million years, the ocean had a pH of approximately 8.2. But, since the beginning of the industrial revolution in the early 1800s, the pH of the ocean has dropped by 0.1 units. Scientists estimate that if CO2 emissions continue at the existing rate, it could reduce ocean pH by another 0.5 units.
How Acidification Causes a Decrease in Calcification?
Acidification leads to a decrease in calcification. This affects just about every life form in the ocean. Calcification is a result of the promotion of calcium carbonate. Too much acid limits the production of calcium bicarbonate. This is bad news for marine life who depend on the calcification process to form shells and plates.
Many marine organisms require calcium carbonate as they are building blocks for their shells and skeletons. If the pH level is too high, growth for other marine organisms becomes limited as they cannot make larger shells to grow into. Coral growth is also inhibited, and there is also more coral bleaching – this reduces the available habitats for marine life too.
The last time that the Earth experienced a notable rise in ocean acidification was over 56 million years ago. It is considered to be the calling card of the Paleocene-Eocene Thermal Maximum era. You don’t read much about that in science classes as its impact was primarily on the ocean’s bottom and ecology located there.
During the same period, the bottom dwellers of the ocean died out, and it is thought that many of our major coral structures also bleached and created a foundation for the coral reefs and underwater structures we explore today.
If you were to compare what happened in the Paleocene-Eocene Thermal Maximum period with what is happening today, the rate is almost 65% higher. This isn’t just that the ocean acidification is occurring at a higher rate, but that it is also occurring at a much faster rate.
Scientists can also see the effects more clearly because of the speed at which the pH levels are changing. Many species are already dying out, coral is bleaching, and the level or depth of photosynthesis within the ocean is slowing down. There is also a higher presence of calcification within the ocean ecosystem with plants and marine life.
Impact of Ocean Acidification
Not all of the impact of ocean acidification is bad. It depends on whose benefit you are assessing when you consider the issue. Some species of marine life thrive in waters with higher pH levels. For man and other marine life – the effects can be devastating.
Positive Impacts
Some marine life forms, such as Sea Stars, thrive under higher pH. Their numbers can increase as their habitat increases in the marine world. If the animals don’t rely heavily on a food chain supported by deep photosynthesis or are prey to animals that do – their population grows.
Negative Impacts
1. Impact on Organisms and Ecosystems
Calcium carbonate minerals are building blocks for skeletons and shells of marine organisms. The continued ocean acidification causes many parts of the ocean to become undersaturated with these minerals resulting in reduced shell and skeleton production and bring changes in assemblages, food webs and ecosystems. It also causes biodiversity loss and changes in biogas production and feedback to climate.
2. Atmospheric Change
Ocean acidification results in increased CO2, bicarbonate ions and acidity. This ultimately causes an increase in atmospheric carbon dioxide.
3. Impact on Fisheries, Aquaculture and Food Security
Ocean acidification presents the potential for devastating effects on the world’s fishing industries. Shellfish (mussels, oysters, sea urchins), coral reefs get affected by the acidity levels of the waters. Not only does the increase in coral bleaching lead to a loss of protective barriers for fishing areas, but the food chain for many fished species is destroyed. That will lead to a loss in fishing production and the potential end of ocean fishing.
4. Distressed Food Chain
Ocean acidification also affects the flow of the food chain. Once one aspect of the food chain is distressed, the rest of the food chain will suffer. When fish are tainted, and in low supply due to a poor food web, local economies will suffer. Fishermen will be unable to produce food for the community, and they will also suffer when fish cannot be sold.
5. Coastal Protection Hampered
Coral reefs protect our coastlines from erosion and flooding. Coral forms the barrier that changes the currents of the water and prevents many storms from reaching land. It supports local economies through tourism and fisheries, and host vastly productive ecosystems. The fact is that the coral bleaches also hold the potential for putting seaside towns and cities at risk.
6. Changes in Climate Regulation
If the coral ceases to grow, it will begin to erode, and that will change coastal weather patterns and ocean impact ratings.
Causes of Ocean Calcification
The two main causes of ocean calcification are natural environmental cycles and man-made pollution.
Natural Causes
The fact that the pH levels in the ocean have changed before is documented, but scientists do not know why this happened. It may be a part of the natural decay cycle of the Earth’s ecosystem.
Man-made Causes
Modern society has become so dependent upon gas, electricity, and a wasteful mindset, which according to scientists, causes the radical increase in carbon emissions from human society that have speed up the rise of the pH levels in the ocean.
1. CO2 Plays a Major Role in Ocean Acidification
The continuous formation of carbon dioxide (CO2) from factories and burning fossil fuels caused carbon emissions to get absorbed into the water and overwhelmed its natural processing of carbon dioxide. This increases carbon dioxide levels in the water, creates acidification and decreases calcification.
2. The Industrial Revolution is Another Cause of Ocean Acidification
Since the beginning of the industrial revolution, the release of CO2 due to man-made activities has increased the amount of CO2 concentrations in the atmosphere.
3. The Lack of Environmentally Friendly Legislation, Laws, and Practices in Our Society
The biochemistry found in oceans is changing and evolving in a negative manner. The negative effect of poor ocean biochemistry will lead to an effect not only on the world’s oceans but to the coastal estuaries and waterways as well.
4. Other Causes of the Rise of the pH Levels
Some of the causes include cement manufacturing, changes in land use, loss of biodiversity, etc.
Solutions to Ocean Acidification
Different solutions are being used to combat ocean acidification. The effectiveness of these solutions remains to be seen, but in an unusually proactive venture, both are being put into place while more discussion is being held on a global level.
1. Limit Man-made Carbon Emissions
This is the center-point of almost all of the climate talks. Governments are capping carbon emission allowances for industries as part of the effort to reduce their impact on ecosystems. There have been several setbacks to these policies, but many industries are finding them to be popular with customers, so are adopting them.
2. Iron Fertilization
By proactively seeding the ocean with iron, scientists hope to create a way to absorb excess carbon dioxide, so the ocean’s systems don’t have to attempt to process it. While this has a good chance of slowing or controlling ocean acidification, the long term effects on the ecosystem are still not known.
3. Policy Options to take action
The conventions such as UN Framework Convention on Climate Change, Conference of the Parties, IPCC, Conference on Sustainable Development (Rio+20), and Convention on Biological Diversity scheduled to address the issue. Regional and local acts, laws and policies need to be formulated to reduce other stresses. Geoengineering can also help in this regard.
Ocean Acidification: A Global Problem
In the end, the real result of the scientific analysis of the trend of ocean acidification is to underscore the need for climate engineering to become a priority. It may not be feasible to limit carbon emissions, given the fast growth rate of manufacturing and human population.
The more people live on earth, the more carbon emissions rise – not just from consumption, but the very act of living too. It may be more realistic to invest in climate engineering proposals, such as iron fertilization, as solutions.
Over the last decade, there has been much interest shown by scientists in studying the impact of ocean acidification. Since the study started a little late, it is impossible to predict how ocean acidification will impact marine life and what will be its consequences on the Earth’s ecosystem.
Scientists and environmental advocates are using education as a way to engage public interest in solving the problem of ocean acidification. Just telling them that fish they like to eat may become more expensive, or extinct, is too far away from the day to make an impact.
Instead, scientists are recasting the information into how it is beginning to change our daily experience of the ocean and marine life. From there, they are promoting support for climate engineering efforts. With the world’s population increasing, making sure the ocean is everyone’s priority is the best way to prevent the potentially disastrous effects of ocean acidification.
References: